By Beatriz T. Martins,a M. Rosário Bronze,a,b,c M. Rita Venturaa*
aITQB NOVA—Instituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, Av. da República, 2780-157 Oeiras, Portugal. rventura@itqb.unl.pt. +351 214469775
bFFULisboa—Faculdade de Farmácia da Universidade de Lisboa, Av. das Forças Armadas, 1649‐019 Lisboa, Portugal.
cIBET—Instituto de Biologia Experimental e Tecnológica, Av. da República, Estação Agronómica Nacional, 2780‐157 Oeiras, Portugal
Virgin olive oil (VOO) is the main fat consumed by the populations from the Mediterranean basin, and it is a valuable source of highly abundant unsaturated fatty acids (representing nearly 98% of the oil chemical composition) and minor components such as fat-soluble vitamins, chlorophylls, phytosterols and phenolic compounds.[1]
When it comes to health-promoting properties, olive oil phenols play a key role, having attracted considerable research interest. Indeed, the phenolic compounds present in this fat have shown to possess a broad spectrum of biological activities, namely, antioxidant, anti-inflammatory, cardioprotective, neuroprotective, anticancer, antidiabetic, antiobesity, antisteatotic, antimicrobial among other effects.
According to numerous investigations, these effects are mostly due to simple phenols such as hydroxytyrosol 1 and tyrosol 2 and the main secoiridoid derivatives such as oleuropein 3, ligstroside 4, oleacein 5 and oleocanthal 6 (Figure 1).[1] This group of phenolic compounds has great potential for therapeutic success in counteracting multifactorial pathologies due to their multiple modes of action.
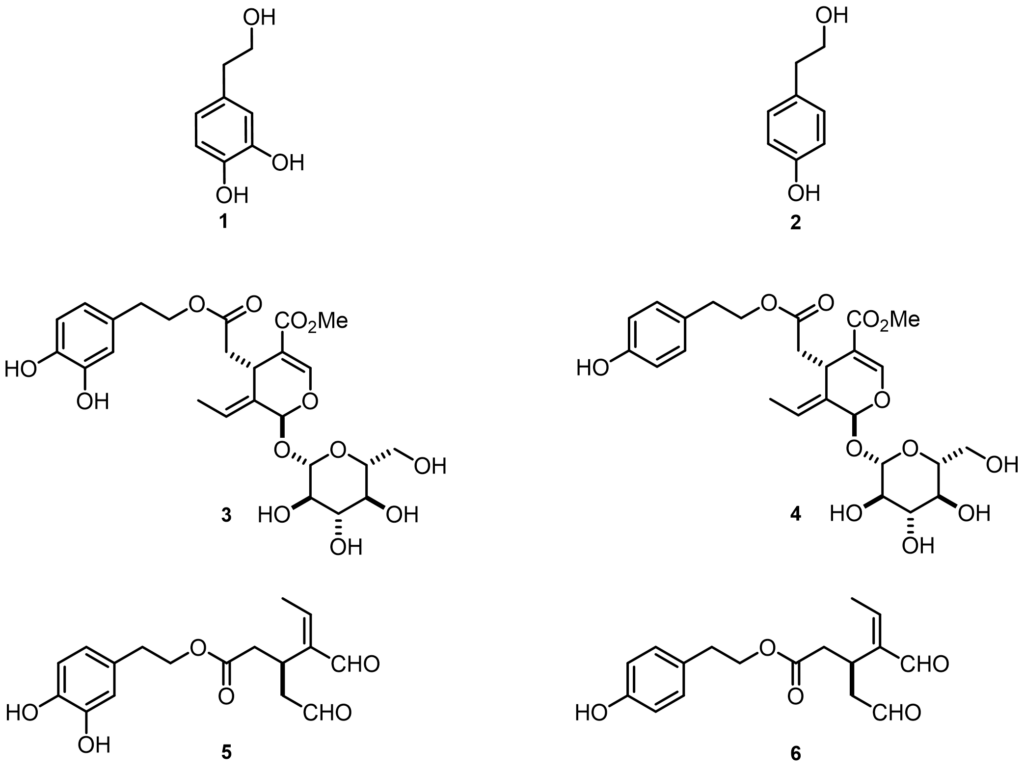
Figure 1 – Chemical structures of hydroxytyrosol 1, tyrosol 2, oleuropein 3, ligstroside 4, oleacein 5 and oleocanthal 6.
Several of the health benefits appointed to VOO phenolic compounds have been extensively reported. However, some of these compounds may not be commercially available, and the amount obtained from their natural sources is small and limited, hampering the studies on the mechanisms underlying their biological activity. In order to contribute to a better knowledge of the bioactivity of these compounds and their metabolites, they must be available in a high pure grade and in sufficient amounts for the assays and their stability must be well known.
As a result of the growing interest in the production of bioactive compounds, the development of innovative extraction and characterisation technologies has been a key factor in the olive oil sector. In this sense, the by-products derived from the processing of Olea europaea L. started to be recovered and reused for several purposes following the circular economy policies. The most used technique to separate the phenolic compounds from agricultural wastewater is by traditional extraction such as the use of organic solvents and filtration processes (membrane). Nonetheless, the amount of secoiridoids represents just a small part of the bioactive compounds of the residues: 15.2%, 0.7% and 3.7% of the bioactive fraction of olive pulp, seeds and olive mill wastewater, respectively.[2] In spite of the advances in this area, the amount of extracted secoiridoids from VOO waste processing is too low to consider their extraction as a possible method for obtaining them in useful quantities. In this sense, chemical synthesis can be considered the best route to obtain bioactive compounds in larger amounts.
Our recent review article covered state-of-the-art chemical synthetic methods to obtain the major olive oil secoiridois oleuropein 3, ligstroside 4, oleacein 5, oleocanthal 6, as well as the synthetic protocols for the preparation of their analogues[3]. These compounds have shown many important biological and pharmacological properties, however there are not many syntheses described for these compounds, compared with other natural compounds.
One of our aims was to standardise the representation of oleuropein 3, ligstroside 4, oleacein 5, oleocanthal 6, emphasising the known natural stereoisomer of each compound, which were sometimes represented in ambiguous ways. In fact, these compounds present a highly functionalised structure, for example, oleuropein 3 and ligstroside 4 possess an E-double bond, two asymmetric centres with S configuration and a beta-glucosidic bond.
Several studies reporting the biological and pharmacological properties of these phenolic compounds were performed with the compounds extracted from VOO or in which the structure of these phenolic compounds was not correctly represented in terms of stereochemistry. Thus, chemical synthesis was very important to confirm the structure of these compounds and their metabolites, especially to determine their stereochemistry. The chemical synthesis also paved the way for the synthesis of non-natural stereoisomers of secoiridoids and other analogues for structure–activity relationship studies in order to modulate their properties for potential therapeutic applications.
We believe that this review has a great interest to all scientists working with olive oil and its components, in many research fields as natural products, pharmaceutical sciences, food sciences, biology, biochemistry, plant sciences, medicinal chemistry and asymmetric synthesis.
We are currently working on the synthesis and biological evaluation of these compounds and a major drawback in the literature search was the lack of a report covering the total chemical syntheses of the main VOO secoiridoids.
Read more at: https://doi.org/10.1021/acs.jafc.2c05349
References
[1] – Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24 (10), 2001−2040.
[2] – Otero, P.; Garcia-Oliveira, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; Garcia-Perez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; Prieto, M. A. Applications of by-products from the olive oil processing: Revalorization strategies based on target molecules and green extraction technologies. Trends Food Sci. Technol. 2021, 116, 1084−1104.
[3] – Martins, B. T.; Bronze, M. R.; Ventura, M. R. Phenolic Compounds from Virgin Olive Oil: Approaches for Their Synthesis and Analogues. J. Agric. Food Chem. 2022, 70, 14109–14128

Beatriz Torgal Martins is a PhD student at the Bioorganic Chemistry Laboratory at Instituto de Tecnologia Química e Biológica António Xavier (ITQB-NOVA) in Oeiras, Portugal. Her PhD focuses on the evaluation of the therapeutic properties of oleocanthal, oleacein and analogues. For this purpose, the design and development of a new efficient asymmetric synthesis of oleocanthal, oleacein is the main aim, as well as the synthesis of metabolites of VOO phenolic compounds. Her project is interdisciplinary at the crossroad between chemistry (synthesis of the compounds and the analytical studies) and biology (in vitro studies, namely metabolism and cell-based assays). Her PhD work intends to be an important step to understanding how this family of compounds can contribute to the health benefits associated with VOO consumption and can open new strategies to the production of new molecules with interest for the pharmaceutical industry.
Rita Ventura is the head of the Bioorganic Chemistry Laboratory at at Instituto de Tecnologia Química e Biológica António Xavier (ITQB-NOVA) in Oeiras, Portugal. R. Ventura’s Lab main research aim is to synthesise biologically active molecules, to modify their structure in order to enhance or modulate their biological properties and to contribute to the understanding of the biological phenomena in which they play a crucial role. This knowledge is important for the design and synthesis of new molecules that can interfere with the molecular mechanisms responsible for pathogenic behaviours, with potential innovative therapeutic applications. Ventura obtained a PhD degree in Chemistry from Universidade Nova de Lisboa in 1999 and graduated in Pharmaceutical Sciences, Faculdade de Farmácia da Universidade de Lisboa in 1995. Ventura has extensive experience in asymmetric total synthesis of natural compounds and analogues with improved biological and therapeutic properties, carbohydrate chemistry, stereoselective organocatalysis and medicinal chemistry.
Maria Rosário Bronze is Senior Scientific Advisor at Food & Health Division at Instituto de Biologia Experimental e Tecnológica (iBET) in Oeiras, Portugal. MRB is also Associate Professor at Faculdade de Farmácia da Universidade de Lisboa and head of Structural Analysis Laboratory at the same institution. Working in Analytical Chemistry since 1986, her PhD in 1999 contributed to the study of food products using capillary electrophoresis. Current research is focused on the quality and the beneficial health effects of food components. Analytical methodologies including mass spectrometry state-of-the-art are used to fully characterize different matrices, from food products, natural extracts or biological fluids. The main food products studied have been olive tree products (olive, olive oil, leaves), cereals as maize, leguminous (faba bean, pea, chickpea, lentils) fruits (apple, grapes, opuntia ficcus), fruit juices and wine, residues from food industries, among others.

